Electrolysis in the Cooling System
Electrolysis in an automotive cooling system can be hazardous to the lifespan of metal and, in particular, aluminum. It is often left unnoticed until it is too late, and leaks have already started to form.
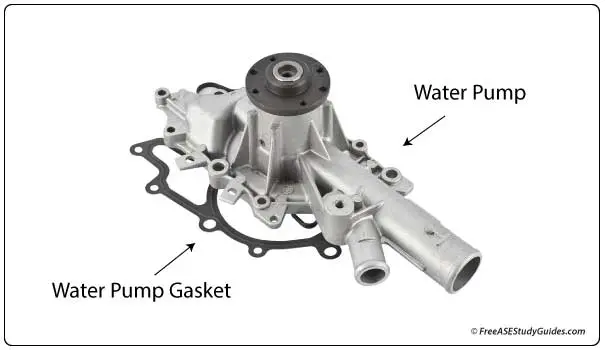
Electrolysis is extremely corrosive and eats away the inside lining of aluminum parts, notably thinner parts like aluminum heater cores. It occurs when two dissimilar metals are joined together in the presence of moisture. It's a chemical or electrical process that corrodes the weaker of the two metals.
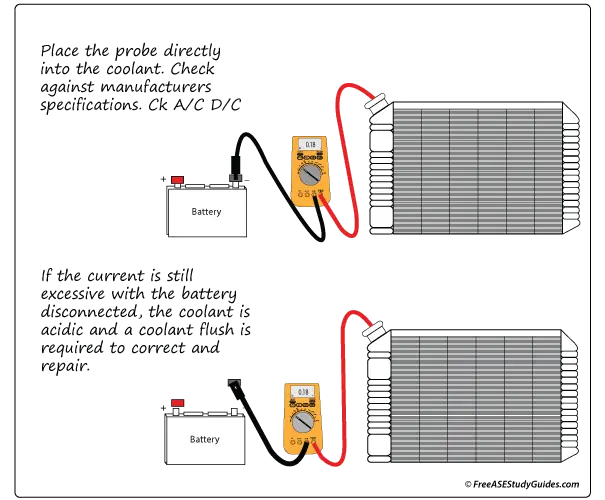
A current of as low as 0.5V can corrode a cast-iron block. It takes much less current to damage an aluminum engine block and many of its components. It's closer to 0.15V to 0.20V. Use both A/C and D/C settings for this test. This problem can happen after adding an aftermarket part or breaking a ground circuit. The part or connection causing the excess current must be found and repaired.

Electrolysis occurs when the engine's coolant breaks down, or the ratio between the coolant and the water is mostly water. As it breaks down, it becomes acidic. It acts as a catalyst for electrical current. A small amount of electrical current can flow through the coolant corroding the lesser metal, often an aluminum part like the water pump or engine coolant outlet.